1906
Dr. Alois Alzheimer first describes "a peculiar disease."
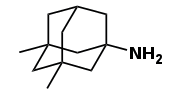
1968
Memantine first synthesized at Lilly.
1984
Beta-amyloid identified by researchers; the chief component of Alzheimer's disease brain plaques and a prime suspect in triggering nerve cell damage.
1986
Tau protein identified by researchers as a key component of tangles — the second pathological hallmark of Alzheimer's disease and another prime suspect in nerve cell degeneration.
1990s
Xanomeline is the first molecule developed by Lilly for the treatment of Alzheimer's disease; program is discontinued following Phase 2 trial.
1996
Donepezil, an acetylcholinesterase inhibitor, is approved.

1990s & 2000s
Development and termination of semagacestat, an inhibitor of the gamma-secretase enzyme.
Early 2000s
Solanezumab, an anti-amyloid antibody, (first-in-human dose) FHD date.

2003
Memantine is FDA approved for moderate to severe dementia of the Alzheimer’s type.
2008
BACE I (Beta-secretase) inhibitor candidate selected.

2010
Lilly acquires Avid Radiopharmaceuticals, Inc., a privately held company developing novel molecular imaging compounds.

2011
Donanemab, an anti-amyloid antibody, FHD date.

2012
First amyloid imaging agent (Amyvid) approved by the FDA to detect the presence of amyloid plaque in the brain.

2012
Lilly announces pooled results from the solanezumab Phase 3 EXPEDITION & EXPEDITION2 trials with data supporting efficacy in mild, but not moderate AD dementia, in a pre-specified secondary analysis.
Additionally, a biomarker sub study showed approximately 20-30% of participants did not have amyloid pathology. The subsequent solanezumab Phase 3 study, EXPEDITION3 was the first to require pathology for inclusion. An AD diagnosis must include a clinical assessment as well as biomarker confirmation of the hallmark neuropathology.
2013
Initiation of EXPEDITION 3 with solanezumab — the first Alzheimer’s disease trial to require evidence of pathology.

2014
Lilly's public-private partnership initiation with National Institute of Aging (NIA) of the first secondary prevention study, A4 Study, in late onset Alzheimer's disease for solanezumab.

2016
Lilly collaborates on Alzheimer's disease Adjunct Diagnostic Tool, a commercially scalable cerebrospinal fluid assay for amyloid-beta 1-42.

2016
Solanezumab does not meet the primary endpoint in the EXPEDITION3 clinical trial; Lilly did not pursue regulatory submissions for the treatment of mild dementia due to Alzheimer's disease.
2017
First Lilly trial using tau PET for screening.

2018
Termination of the BACE programs.

2018
Lilly begins Phase 2 clinical trial of zagotenemab in patients with early symptomatic Alzheimer's disease.

2020
First tau imaging agent (Tauvid) approved by the FDA to detect the presence of tau tangles in the brain.

2020
First Lilly trial using blood-based biomarker for screening.

2021
Lilly completes enrollment for Phase 3 TRAILBLAZER-ALZ 2 trial of donanemab. Roughly 1,800 participants were enrolled.

2021
FDA grants breakthrough therapy designation for donanemab.

2021
Zagotenemab does not meet the primary endpoint in phase 2 trial; Lilly ends development.

2021
Lilly initiates first oral, brain-penetrant, once daily pill in Phase 2 anti-tau therapy study.
2022
Completion of donanemab submission for accelerated approval.

2022
Lilly begins Phase 3 trial of remternetug, an investigational monoclonal antibody, in patients with mild cognitive impairment due to Alzheimer's disease.
